Page 33 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 33
Krisman
amino acid residues. The sequence of a amino 442. (d) In organic chemistry, keto-enol tautom-
acids in a protein is defined by the sequence of erism refers to a chemical equilibrium between
a gene, which is encoded in the genetic code. a keto form (a ketone) and an enol (an alde-
hyde) form. The enol and keto forms are said to
440. (d) Denaturation is a process in which be tautomers of each other. The interconversion
proteins or nucleic acids lose their structure (ter- of the two forms involves the movement of a
tiary structure) by application of some external proton and the shifting of bonding electrons;
stress or compound, for example, treatment of hence, the isomerism qualifies as tautomerism.
proteins with strong acids or bases, high
concentrations of inorganic salts, organic sol- Purine Tautomeric Forms:
vents (e.g. alcohol or chloroform), or heat. If
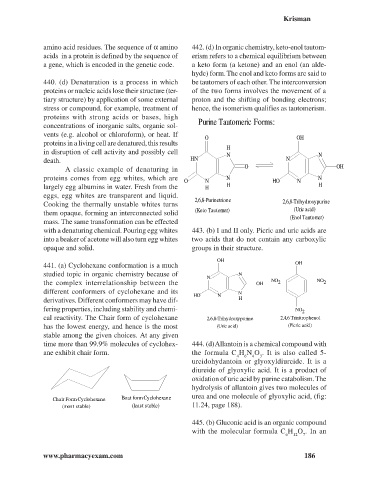
O OH
proteins in a living cell are denatured, this results
H
in disruption of cell activity and possibly cell N N
death. HN N
O OH
A classic example of denaturing in
proteins comes from egg whites, which are N N
O N H O N
largely egg albumins in water. Fresh from the H H H
eggs, egg whites are transparent and liquid.
2,6,8-Purinetrione 2,6,8-Trihydroxypurine
Cooking the thermally unstable whites turns
(Keto Tautomer) (Uric acid)
them opaque, forming an interconnected solid
(Enol Tautomer)
mass. The same transformation can be effected
with a denaturing chemical. Pouring egg whites 443. (b) I and II only. Picric and uric acids are
into a beaker of acetone will also turn egg whites two acids that do not contain any carboxylic
opaque and solid. groups in their structure.
OH
441. (a) Cyclohexane conformation is a much OH
studied topic in organic chemistry because of N
N
the complex interrelationship between the OH NO 2 NO 2
different conformers of cyclohexane and its N
H O N
derivatives. Different conformers may have dif- H
fering properties, including stability and chemi- NO 2
cal reactivity. The Chair form of cyclohexane 2,6,8-Trihydroxypurine 2,4,6 Trinitrophenol
has the lowest energy, and hence is the most (Uric acid) (Picric acid)
stable among the given choices. At any given
time more than 99.9% molecules of cyclohex- 444. (d) Allantoin is a chemical compound with
ane exhibit chair form. the formula C H N O . It is also called 5-
4 6 4 3
ureidohydantoin or glyoxyldiureide. It is a
diureide of glyoxylic acid. It is a product of
oxidation of uric acid by purine catabolism. The
hydrolysis of allantoin gives two molecules of
Chair Form Cyclohexane Boat form Cyclohexane urea and one molecule of glyoxylic acid, (fig:
(most stable) (least stable) 11.24, page 188).
445. (b) Gluconic acid is an organic compound
with the molecular formula C H O . In an
6 12 7
www.pharmacyexam.com 186