Page 28 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 28
Krisman
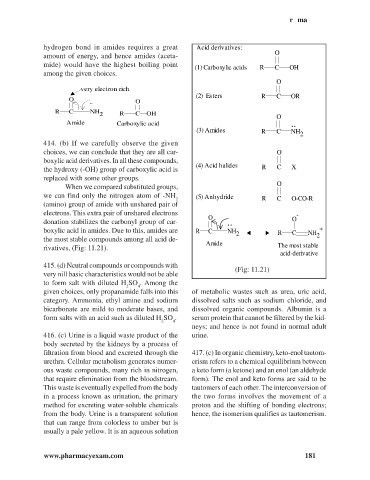
hydrogen bond in amides requires a great Acid derivatives:
O
amount of energy, and hence amides (aceta-
mide) would have the highest boiling point
(1) Carboxylic acids R C OH
among the given choices.
O
very electron rich
(2) Esters R C OR
O
.. O
R C NH
2 R C OH O
Amide Carboxylic acid ..
(3) Amides R C NH
2
414. (b) If we carefully observe the given
choices, we can conclude that they are all car- O
boxylic acid derivatives. In all these compounds,
(4) Acid halides R C X
the hydroxy (-OH) group of carboxylic acid is
replaced with some other groups.
O
When we compared substituted groups,
we can find only the nitrogen atom of -NH (5) Anhydride
2 R C O-CO-R
(amino) group of amide with unshared pair of
electrons. This extra pair of unshared electrons -
O O
donation stabilizes the carbonyl group of car- ..
boxylic acid in amides. Due to this, amides are R C NH +
2 R C NH 2
the most stable compounds among all acid de-
Amide
rivatives, (Fig: 11.21). The most stable
acid-derivative
415. (d) Neutral compounds or compounds with
(Fig: 11.21)
very nill basic characteristics would not be able
to form salt with diluted H SO . Among the
2 4
given choices, only propanamide falls into this of metabolic wastes such as urea, uric acid,
category. Ammonia, ethyl amine and sodium dissolved salts such as sodium chloride, and
bicarbonate are mild to moderate bases, and dissolved organic compounds. Albumin is a
form salts with an acid such as diluted H SO . serum protein that cannot be filtered by the kid-
2 4
neys; and hence is not found in normal adult
416. (c) Urine is a liquid waste product of the urine.
body secreted by the kidneys by a process of
filtration from blood and excreted through the 417. (c) In organic chemistry, keto-enol tautom-
urethra. Cellular metabolism generates numer- erism refers to a chemical equilibrium between
ous waste compounds, many rich in nitrogen, a keto form (a ketone) and an enol (an aldehyde
that require elimination from the bloodstream. form). The enol and keto forms are said to be
This waste is eventually expelled from the body tautomers of each other. The interconversion of
in a process known as urination, the primary the two forms involves the movement of a
method for excreting water-soluble chemicals proton and the shifting of bonding electrons;
from the body. Urine is a transparent solution hence, the isomerism qualifies as tautomerism.
that can range from colorless to amber but is
usually a pale yellow. It is an aqueous solution
www.pharmacyexam.com 181