Page 36 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 36
Krisman
Therefore the correct order should be: 459. (a) In difluorobenzene, the fluorine atom
is highly electronegative compared to carbon.
Phenol > Benzene > Chlorobenzene > Nitroben- Since fluorine has a strong electron withdraw-
zene ing power it pulls out electrons from the carbon
atom, and makes it positively charged where
Please remember the following chart to answer itself possesses a negative charge. This differ-
this type of question: ence in charge on both ends of a bond make the
bond polarized, and hence induces dipole mo-
Strongly activating substituents: ment. The magnitude of dipole moment depends
on the distance between two atoms, an electron
-OH, -OR, -NH , -NHR, -NR withdrawing power of an atom and the direction
2 2
of dipole moment. In para position, dipole mo-
Moderate or weakly activating substituents: ment induced by both fluorine atoms cancel out
each other’s effects. Compared to a meta
-CH , -C H , -R (any alkyl group) position, an ortho position provides synergistic
3 2 5
dipole effects induced by two fluorine atoms.
Deactivating substituents: Therefore, o-difluorobenzene would have the
most dipole moment whereas p-difluorobenzene
-F, -Cl, -Br, -I would have the least dipole moment.
Strongly deactivating substituents: 460. (b) Saccharin is an artificial sweetener. The
basic substance, benzoic sulfinide, has
-NO , -CHO, -COR, -SO H, -CN, -COOH effectively no food energy and is much sweeter
2 3
than sucrose (about 500 times), but has an un-
456. (a) Among the given choices, aniline pleasant bitter or metallic aftertaste, especially
should undergo substitution reactions faster than at high concentrations. Saccharin is used to
benzene (same logic as the answer 455). sweeten products such as drinks, candies, medi-
cines and toothpaste. It is widely marketed as a
457. (c) Since the alkyl group activates the ring soluble sodium salt of saccharin.
toward electronic substitution, ethylbenzene
will undergo the nitration most rapidly among 461. (a) Sulfanilamide is an antibacterial drug.
the given choices. It is the compound from which other sulfa drugs
are derived. Sulfanilamide and its derivatives
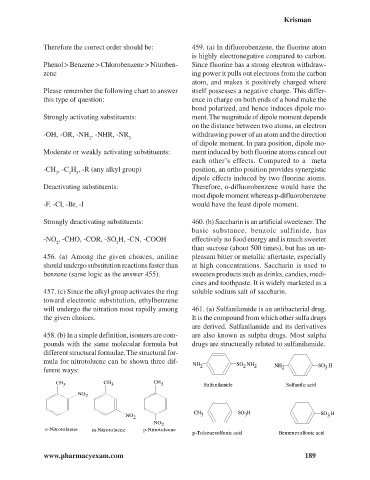
458. (b) In a simple definition, isomers are com- are also known as sulpha drugs. Most sulpha
pounds with the same molecular formula but drugs are structurally related to sulfanilamide.
different structural formulae. The structural for-
mula for nitrotoluene can be shown three dif-
NH 2 SO NH 2 NH SO H
2
ferent ways: 2 3
CH CH CH
3 3 3 Sulfanilamide Sulfanilic acid
NO
2
CH SO H SO H
NO 2 3 3 3
NO
2
o-Nitrotoluene m-Nitrotoluene p-Nitrotoluene
p-Toluenesulfonic acid Benzenesulfonic acid
www.pharmacyexam.com 189