Page 31 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 31
Krisman
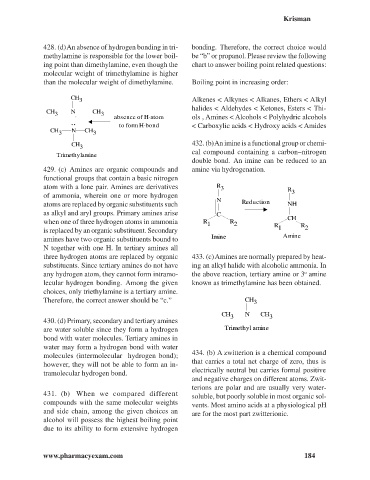
428. (d) An absence of hydrogen bonding in tri- bonding. Therefore, the correct choice would
methylamine is responsible for the lower boil- be “b” or propanol. Please review the following
ing point than dimethylamine, even though the chart to answer boiling point related questions:
molecular weight of trimethylamine is higher
than the molecular weight of dimethylamine. Boiling point in increasing order:
CH
3 Alkenes < Alkynes < Alkanes, Ethers < Alkyl
halides < Aldehydes < Ketones, Esters < Thi-
CH N CH
3 .. 3 absence of H-atom ols , Amines < Alcohols < Polyhydric alcohols
..
to form H-bond < Carboxylic acids < Hydroxy acids < Amides
CH N CH
3 3
CH 432. (b) An imine is a functional group or chemi-
3
cal compound containing a carbon–nitrogen
Trimethylamine
double bond. An imine can be reduced to an
429. (c) Amines are organic compounds and amine via hydrogenation.
functional groups that contain a basic nitrogen
atom with a lone pair. Amines are derivatives R 3 R
of ammonia, wherein one or more hydrogen 3
N Reduction
atoms are replaced by organic substituents such NH
as alkyl and aryl groups. Primary amines arise C
when one of three hydrogen atoms in ammonia R 1 R 2 R CH R
is replaced by an organic substituent. Secondary 1 2
amines have two organic substituents bound to Imine Amine
N together with one H. In tertiary amines all
three hydrogen atoms are replaced by organic 433. (c) Amines are normally prepared by heat-
substituents. Since tertiary amines do not have ing an alkyl halide with alcoholic ammonia. In
o
any hydrogen atom, they cannot form intramo- the above reaction, tertiary amine or 3 amine
lecular hydrogen bonding. Among the given known as trimethylamine has been obtained.
choices, only triethylamine is a tertiary amine.
Therefore, the correct answer should be “c.” CH 3
CH 3 N CH 3
430. (d) Primary, secondary and tertiary amines
are water soluble since they form a hydrogen Trimethyl amine
bond with water molecules. Tertiary amines in
water may form a hydrogen bond with water
434. (b) A zwitterion is a chemical compound
molecules (intermolecular hydrogen bond);
that carries a total net charge of zero, thus is
however, they will not be able to form an in-
electrically neutral but carries formal positive
tramolecular hydrogen bond.
and negative charges on different atoms. Zwit-
terions are polar and are usually very water-
431. (b) When we compared different
soluble, but poorly soluble in most organic sol-
compounds with the same molecular weights vents. Most amino acids at a physiological pH
and side chain, among the given choices an are for the most part zwitterionic.
alcohol will possess the highest boiling point
due to its ability to form extensive hydrogen
www.pharmacyexam.com 184