Page 30 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 30
Krisman
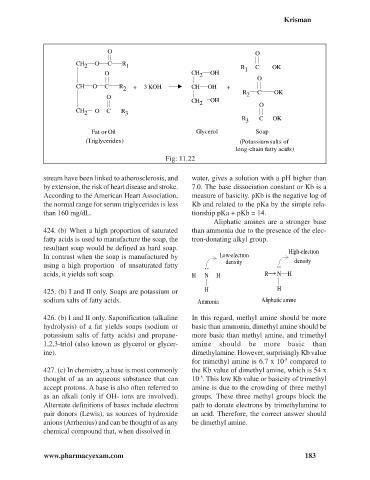
O O
CH O C R
2 1 R 1 C OK
O CH 2 OH
O
CH O C R + 3 KOH CH OH +
2
R 2 C OK
O
CH OH
2 O
CH 2 O C R 3
R C OK
3
Fat or Oil Glycerol Soap
(Triglycerides) (Potassium salts of
long-chain fatty acids)
Fig: 11.22
stream have been linked to atherosclerosis, and water, gives a solution with a pH higher than
by extension, the risk of heart disease and stroke. 7.0. The base dissociation constant or Kb is a
According to the American Heart Association, measure of basicity. pKb is the negative log of
the normal range for serum triglycerides is less Kb and related to the pKa by the simple rela-
than 160 mg/dL. tionship pKa + pKb = 14.
Aliphatic amines are a stronger base
424. (b) When a high proportion of saturated than ammonia due to the presence of the elec-
fatty acids is used to manufacture the soap, the tron-donating alkyl group.
resultant soap would be defined as hard soap. High-electron
In contrast when the soap is manufactured by Low-electron density
using a high proportion of unsaturated fatty .. density ..
acids, it yields soft soap. H N H R N H
425. (b) I and II only. Soaps are potassium or H H
sodium salts of fatty acids. Ammonia Aliphatic amine
426. (b) I and II only. Saponification (alkaline In this regard, methyl amine should be more
hydrolysis) of a fat yields soaps (sodium or basic than ammonia, dimethyl amine should be
potassium salts of fatty acids) and propane- more basic than methyl amine, and trimethyl
1,2,3-triol (also known as glycerol or glycer- amine should be more basic than
ine). dimethylamine. However, surprisingly Kb value
-5
for trimethyl amine is 6.7 x 10 compared to
427. (c) In chemistry, a base is most commonly the Kb value of dimethyl amine, which is 54 x
-5
thought of as an aqueous substance that can 10 . This low Kb value or basicity of trimethyl
accept protons. A base is also often referred to amine is due to the crowding of three methyl
as an alkali (only if OH- ions are involved). groups. These three methyl groups block the
Alternate definitions of bases include electron path to donate electrons by trimethylamine to
pair donors (Lewis), as sources of hydroxide an acid. Therefore, the correct answer should
anions (Arrhenius) and can be thought of as any be dimethyl amine.
chemical compound that, when dissolved in
www.pharmacyexam.com 183