Page 38 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 38
Krisman
parts of a single molecule (intramolecularly), phenoxy oxygen is then able to form strong
as we see in o-nitrophenol. The hydrogen bond hydrogen bonds with water molecules. This also
is a very strong fixed dipole-dipole van der explains the higher boiling point of phenols
Waals-Keesom force, but weaker than covalent, compared to the boiling points of alcohols with
ionic and metallic bonds. The hydrogen bond comparable molecular weights.
is somewhere between a covalent bond and an
electrostatic intermolecular attraction. Intermo- 470. (a) The boiling point of any given com-
lecular hydrogen bonding is responsible for the pound is highly dependant on its ability to form
high boiling point of water (100 °C). a hydrogen bond with water molecules. The
compound more capable of forming hydrogen
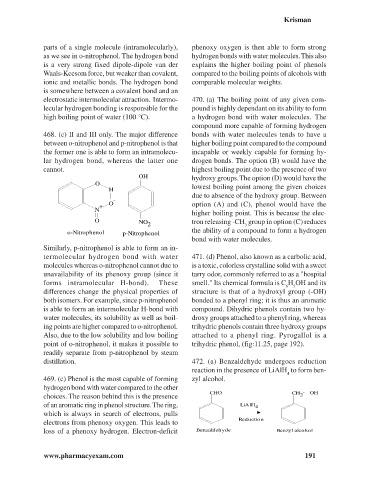
468. (c) II and III only. The major difference bonds with water molecules tends to have a
between o-nitrophenol and p-nitrophenol is that higher boiling point compared to the compound
the former one is able to form an intramolecu- incapable or weekly capable for forming hy-
lar hydrogen bond, whereas the latter one drogen bonds. The option (B) would have the
cannot. highest boiling point due to the presence of two
OH hydroxy groups. The option (D) would have the
O lowest boiling point among the given choices
H
due to absence of the hydroxy group. Between
-
O option (A) and (C), phenol would have the
+
N
higher boiling point. This is because the elec-
O NO tron releasing -CH group in option (C) reduces
2 3
o-Nitrophenol p-Nitrophenol the ability of a compound to form a hydrogen
bond with water molecules.
Similarly, p-nitrophenol is able to form an in-
termolecular hydrogen bond with water 471. (d) Phenol, also known as a carbolic acid,
molecules whereas o-nitrophenol cannot due to is a toxic, colorless crystalline solid with a sweet
unavailability of its phenoxy group (since it tarry odor, commonly referred to as a "hospital
forms intramolecular H-bond). These smell." Its chemical formula is C H OH and its
6 5
differences change the physical properties of structure is that of a hydroxyl group (-OH)
both isomers. For example, since p-nitrophenol bonded to a phenyl ring; it is thus an aromatic
is able to form an intermolecular H-bond with compound. Dihydric phenols contain two hy-
water molecules, its solubility as well as boil- droxy groups attached to a phenyl ring, whereas
ing points are higher compared to o-nitrophenol. trihydric phenols contain three hydroxy groups
Also, due to the low solubility and low boiling attached to a phenyl ring. Pyrogallol is a
point of o-nitrophenol, it makes it possible to trihydric phenol, (fig:11.25, page 192).
readily separate from p-nitrophenol by steam
distillation. 472. (a) Benzaldehyde undergoes reduction
reaction in the presence of LiAlH to form ben-
4
469. (c) Phenol is the most capable of forming zyl alcohol.
hydrogen bond with water compared to the other
CHO CH OH
choices. The reason behind this is the presence 2
of an aromatic ring in phenol structure. The ring, LiAlH 4
which is always in search of electrons, pulls
Reduction
electrons from phenoxy oxygen. This leads to
loss of a phenoxy hydrogen. Electron-deficit Benzaldehyde Benzyl alcohol
www.pharmacyexam.com 191