Page 39 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 39
Krisman
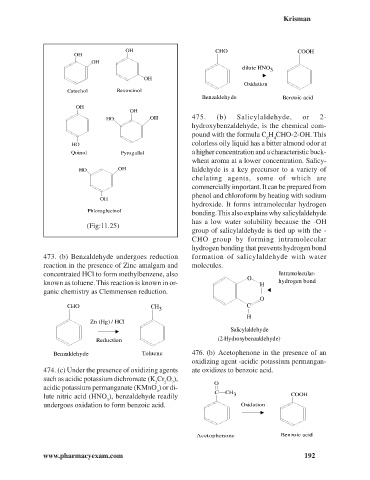
OH CHO COOH
OH
OH
dilute HNO 3
OH
Oxidation
Catechol Resorcinol
Benzaldehyde Benzoic acid
OH
OH
H O OH 475. (b) Salicylaldehyde, or 2-
hydroxybenzaldehyde, is the chemical com-
pound with the formula C H CHO-2-OH. This
6 4
H O colorless oily liquid has a bitter almond odor at
Quinol Pyrogallol a higher concentration and a characteristic buck-
wheat aroma at a lower concentration. Salicy-
H O OH laldehyde is a key precursor to a variety of
chelating agents, some of which are
commercially important. It can be prepared from
phenol and chloroform by heating with sodium
OH
hydroxide. It forms intramolecular hydrogen
Phloroglucinol
bonding. This also explains why salicylaldehyde
has a low water solubility because the -OH
(Fig:11.25)
group of salicylaldehyde is tied up with the -
CHO group by forming intramolecular
hydrogen bonding that prevents hydrogen bond
473. (b) Benzaldehyde undergoes reduction formation of salicylaldehyde with water
reaction in the presence of Zinc amalgam and molecules.
concentrated HCl to form methylbenzene, also Intramolecular-
O
known as toluene. This reaction is known in or- H hydrogen bond
ganic chemistry as Clemmensen reduction.
O
CHO CH C
3
H
Zn (Hg) / HCl
Salicylaldehyde
Reduction (2-Hydroxybenzaldehyde)
Benzaldehyde Toluene 476. (b) Acetophenone in the presence of an
oxidizing agent -acidic potassium permangan-
474. (c) Under the presence of oxidizing agents ate oxidizes to benzoic acid.
such as acidic potassium dichromate (K Cr O ),
2 2 7 O
acidic potassium permanganate (KMnO ) or di-
4 C CH
lute nitric acid (HNO ), benzaldehyde readily 3 COOH
3
undergoes oxidation to form benzoic acid. Oxidation
Acetophenone Benzoic acid
www.pharmacyexam.com 192