Which of the following is/are considered Diabetes Risk Factor(s)? [Select ALL THAT APPLY].
a. Physical inactivity.
b. Women who delivered a baby greater than 9 lb.
c. First-degree relative with diabetes.
d. High-risk race/ethnicity
e. Hypertension greater or equal to 140/90 mm Hg
A. a
B. b
C. c
D. d
E. e
Which of the following is/are considered Diabetes Risk Factor(s)? [Select ALL THAT APPLY].
a. Physical inactivity.
b. Women who delivered a baby greater than 9 lb.
c. First-degree relative with diabetes.
d. High-risk race/ethnicity
e. Hypertension greater or equal to 140/90 mm Hg
A. a
B. b
C. c
D. d
E. e
Answer (a,b,c,d,e). Diabetes Risk Factors:
1. Physical inactivity.
2. First-degree relative with diabetes.
3. Women who delivered a baby >9 lb or were diagnosed with GDM.
4. High-risk race/ethnicity.
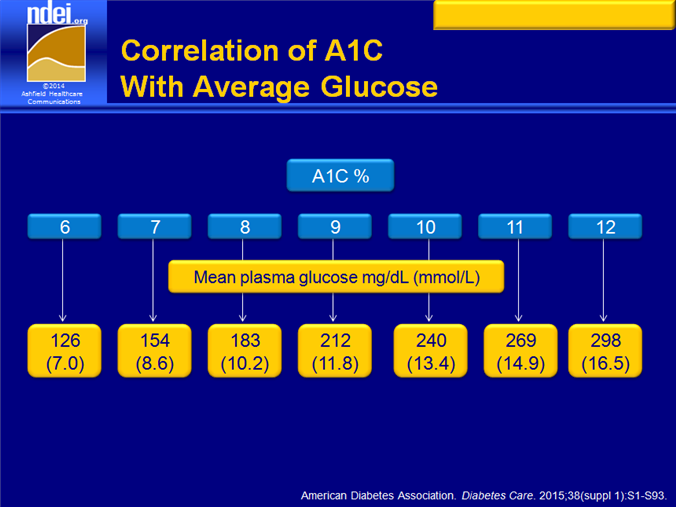
5. A1C greater or equal to 5.7%, Impaired Glucose Tolerance (IGT), or Impaired Fasting Glucose (IFG) on previous testing.
6. Hypertension ( greater or equal to 140/90 mm Hg or on therapy).
7. HDL-C <35 mg/dL plus or minus TG >250 mg/dL.
Try Naplex QBank. www.pharmacyexam.com