Page 45 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 45
Krisman
497. (c) The Friedel-Crafts reactions are a set orbitals allow cyclic delocalization of p elec-
of reactions developed by Charles Friedel and trons. There are few important criteria to be re-
James Crafts in 1877. There are two main types membered to determine whether the compound
of Friedel-Crafts reactions: alkylation reactions is aromatic or non-aromatic.
and acylation reactions. Here we will discuss
Friedel-Crafts acylation. 1. A compound should be cyclic and
Friedel-Crafts acylation is the acylation planar.
of aromatic rings (benzene) with an acyl
chloride (CH COCl) using a strong Lewis acid 2. Each atom in an aromatic ring should
3
catalyst (AlCl ). Friedel-Crafts acylation is also have a p-orbital. These p orbitals must
3
possible with acid anhydrides. This reaction has be parallel so that a continuous overlap
several advantages over the alkylation reaction. is possible around the ring.
Due to the electron-withdrawing effect of the
carbonyl group, the ketone (acetophenone) 3. The cyclic p molecular orbital formed
produced under the reaction is always less re- by the overlap of p orbitals must contain
active than the original molecule (benzene), so (4n + 2) p electrons, where n is equal to
multiple acylations do not occur. integer 0, 1, 2 etc. This is known as
Huckel Rule.
O
CH CO Cl C CH 3
3
In order for a compound to be an aro-
AlCl + HCl matic, these three rules must be followed. Let’s
3
discuss benzene and cycloheptatriene to get a
Benzene Acetophenone
better idea.
498. (b) Organometallic compounds are those
compounds that contain at least one carbon-
metal bond where metals can be Mg, Li, Pb,
Zn, etc, for example, ethyllithium (C H Li).
2 5
Organomagnesium halides are known as Grig-
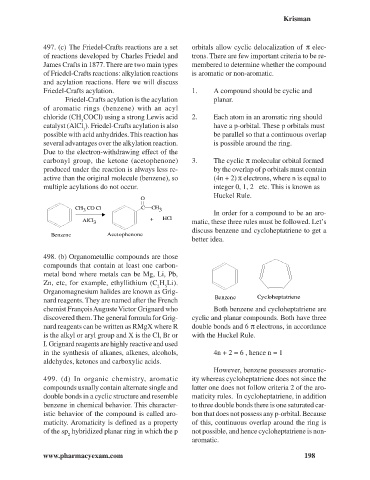
Benzene Cycloheptatriene
nard reagents. They are named after the French
chemist François Auguste Victor Grignard who Both benzene and cycloheptatriene are
discovered them. The general formula for Grig- cyclic and planar compounds. Both have three
nard reagents can be written as RMgX where R double bonds and 6 p electrons, in accordance
is the alkyl or aryl group and X is the Cl, Br or with the Huckel Rule.
I. Grignard reagents are highly reactive and used
in the synthesis of alkanes, alkenes, alcohols, 4n + 2 = 6 , hence n = 1
aldehydes, ketones and carboxylic acids.
However, benzene possesses aromatic-
499. (d) In organic chemistry, aromatic ity whereas cycloheptatriene does not since the
compounds usually contain alternate single and latter one does not follow criteria 2 of the aro-
double bonds in a cyclic structure and resemble maticity rules. In cycloheptatriene, in addition
benzene in chemical behavior. This character- to three double bonds there is one saturated car-
istic behavior of the compound is called aro- bon that does not possess any p-orbital. Because
maticity. Aromaticity is defined as a property of this, continuous overlap around the ring is
of the sp hybridized planar ring in which the p not possible, and hence cycloheptatriene is non-
2
aromatic.
www.pharmacyexam.com 198