Page 8 - FPGEE Medicinal and Organic Chemistry Q&A Book
P. 8
Krisman
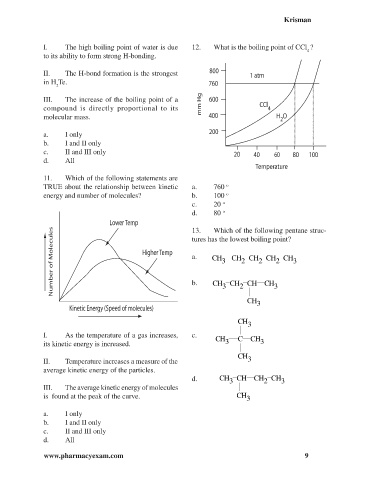
I. The high boiling point of water is due 12. What is the boiling point of CCl ?
4
to its ability to form strong H-bonding.
II. The H-bond formation is the strongest
in H Te.
2
III. The increase of the boiling point of a
compound is directly proportional to its
molecular mass.
a. I only
b. I and II only
c. II and III only
d. All
11. Which of the following statements are
TRUE about the relationship between kinetic a. 760 o
energy and number of molecules? b. 100 o
c. 20 o
d. 80 o
13. Which of the following pentane struc-
tures has the lowest boiling point?
a. CH 3 CH 2 CH 2 CH 2 CH 3
b. CH CH CH CH
3 2 3
CH
3
CH
3
I. As the temperature of a gas increases, c.
CH C CH
its kinetic energy is increased. 3 3
CH
II. Temperature increases a measure of the 3
average kinetic energy of the particles.
d. CH 3 CH CH 2 CH 3
III. The average kinetic energy of molecules
is found at the peak of the curve. CH 3
a. I only
b. I and II only
c. II and III only
d. All
www.pharmacyexam.com 9